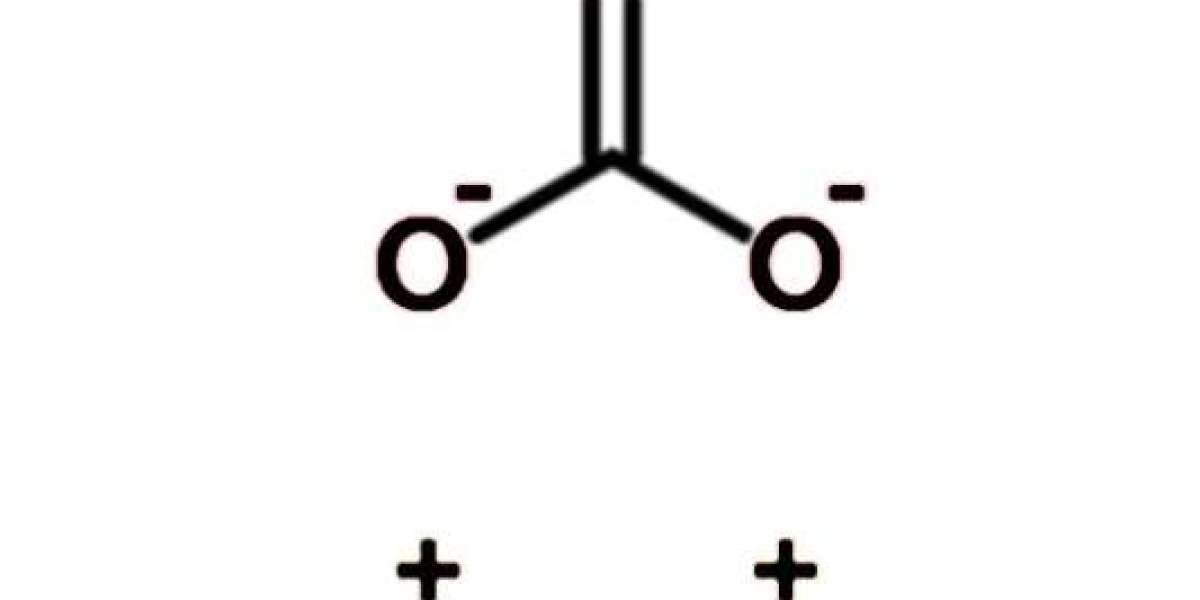
Sodium carbonate, also known as soda ash or washing soda, is a white, odorless powder that is commonly used in various industries, including the chemical, glass, and textile industries. It is also used in laboratories as a pH regulator, a cleaning agent, and a buffer solution. However, to ensure its purity and sterility, sodium carbonate needs to be extracted from its raw form. In this blog post, SACH BIOTECH will provide a comprehensive guide on how to extract white sodium carbonate sterile powder for laboratory use.
Step 1: Obtain the raw material
The first step in extracting sodium carbonate is to obtain the raw material. Sodium carbonate can be found in various forms, including natural deposits, synthetic production, and waste streams. However, for laboratory use, it is recommended to use synthetic sodium carbonate, which is produced by the Solvay process. Synthetic sodium carbonate is preferred because it is more pure and has a consistent composition.
Step 2: Purify the raw material
Once you have obtained the raw material, the next step is to purify it. The purification process involves removing any impurities that may be present in the raw material. Impurities can include other salts, metals, and organic compounds. The purification process can be done using various methods, including filtration, precipitation, and crystallization.
Filtration is a common method used to purify sodium carbonate. It involves passing the raw material through a filter to remove any impurities. The filter can be made of various materials, including paper, cloth, or a membrane. The choice of filter material will depend on the size and nature of the impurities.
Precipitation is another method used to purify sodium carbonate. It involves adding a chemical reagent to the raw material to form a precipitate. The precipitate can then be separated from the solution using filtration or centrifugation. The choice of reagent will depend on the nature of the impurities.
Crystallization is a more complex method used to purify sodium carbonate. It involves dissolving the raw material in a solvent and then allowing it to crystallize. The crystals can then be separated from the solution using filtration or centrifugation. Crystallization is a more time-consuming process, but it can produce a more pure product.
Step 3: Sterilize the purified material
Once the raw material has been purified, the next step is to sterilize it. Sterilization is important to ensure that the sodium carbonate is free from any microorganisms that may be present. Sterilization can be done using various methods, including autoclaving, filtration, and irradiation.
Autoclaving is a common method used to sterilize sodium carbonate. It involves subjecting the purified material to high pressure and temperature to kill any microorganisms that may be present. Autoclaving is a reliable method of sterilization, but it can be time-consuming.
Filtration is another method used to sterilize sodium carbonate. It involves passing the purified material through a filter with a pore size small enough to trap any microorganisms. Filtration is a quick and efficient method of sterilization, but it may not be effective against all types of microorganisms.
Irradiation is a less common method used to sterilize sodium carbonate. It involves exposing the purified material to ionizing radiation to kill any microorganisms that may be present. Irradiation is a quick and efficient method of sterilization, but it may not be suitable for all types of sodium carbonate.
![[CAS: 497-19-8] Sodium carbonate sterile powder [CAS: 497-19-8] Sodium carbonate sterile powder](https://img01.71360.com/w3/jjo137/20231107/21b35796f6bd26b005a87efb5be917da.webp?ct=webp)
Step 4: Dry the sterilized material
Once the purified material has been sterilized, the next step is to dry it. Drying is important to remove any moisture that may be present in the sodium carbonate. Moisture can affect the purity and stability of the sodium carbonate. Drying can be done using various methods, including air-drying, vacuum drying, and freeze-drying.
Air-drying is a simple method used to dry sodium carbonate. It involves spreading the sterilized material on a clean surface and allowing it to dry naturally. Air-drying is a quick and easy method, but it may not be suitable for all types of sodium carbonate.
Vacuum drying is a more complex method used to dry sodium carbonate. It involves subjecting the sterilized material to low pressure and temperature to remove any moisture. Vacuum drying is a more efficient method than air-drying, but it requires specialized equipment.
Freeze-drying is a more advanced method used to dry sodium carbonate. It involves freezing the sterilized material and then subjecting it to low pressure and temperature to remove any moisture. Freeze-drying is the most efficient method of drying, but it requires specialized equipment and is more time-consuming.
Step 5: Package the dried material
Once the sodium carbonate has been dried, the final step is to package it. Packaging is important to protect the sodium carbonate from contamination and to ensure its stability. Packaging can be done using various materials, including glass, plastic, and metal.
Glass is a common material used to package sodium carbonate. Glass is inert and does not react with sodium carbonate, making it an ideal material for packaging. Glass containers can be sealed with airtight caps to prevent contamination.
Plastic is another material used to package sodium carbonate. Plastic containers are lightweight and easy to handle, making them a popular choice for packaging. However, plastic can react with sodium carbonate, so it is important to choose a plastic that is compatible with sodium carbonate.
Metal is a less common material used to package sodium carbonate. Metal containers are durable and can provide a high level of protection against contamination. However, metal can react with sodium carbonate, so it is important to choose a metal that is compatible with sodium carbonate.
Conclusion
In conclusion, extracting white sodium carbonate sterile powder for laboratory use requires several steps, including purifying the raw material, sterilizing the purified material, drying the sterilized material, and packaging the dried material. Each step is important to ensure the purity and sterility of the sodium carbonate. By following the steps outlined in this guide, you can extract high-quality sodium carbonate for your laboratory needs.